Talk about precision gene editing.
Scientists from Harvard University have just unveiled a new gene editor that uses the revolutionary CRISPR-Cas9 technology to target and change a single letter in a string of DNA bases — no cutting necessary.
Considering that there are billions of letters in the human genome, converting one letter to another may not sound like much. But tens of thousands of human diseases can be traced to these tiny mistakes, scientists say.
If traditional gene editing is like taking a pair of molecular scissors to a DNA strand to alter a genome, then the new technique, known as base editing, is like using a pencil and eraser, scientists say.
Both methods have their place.
“If your task is to cut and paste something, then you need scissors. If your task is to fix just a single letter, a pencil is best,” said David Liu, a chemical biologist at Harvard University who led the work.

The first base editor was described by Liu’s group last year. At that time, however, they could only use the technology to turn the base cytosine (known in the DNA alphabet as C) into a base that acts like a thymine (known as T).
In the new study, published Wednesday in the journal Nature, the authors present a second base editor that can convert the base adenine (A) into the base inosine (I), which acts like guanine (G).
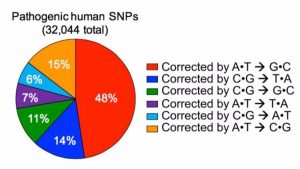
[Image Caption: Of the tens of thousands of human diseases that have been traced to a mistake in a single letter of DNA coding, nearly half could be fixed by changing an A to a G. (David Liu et al, Nature)]
The new work is significant because it will allow scientists to use base editing to address many more single-letter mutations than was previously possible, said Krishanu Saha, a biomedical engineer at the University of Wisconsin Madison who was not involved with the research.
“This is another nice example of using protein engineering to diversify the types of edits that the CRISPR system can accomplish,” he said.
There are 3 billion base pairs in the human genome, and a mistake or mutation in just one single letter can have a significant impact on a person’s health.
Of more than 50,000 genetic changes currently known to be associated with disease in humans, 32,000 of those are caused by the simple swap of one base pair for another, Liu said.
The group’s first base editing tool, which had the effect of converting a C to a T, has the potential to correct 14% of human diseases associated with a single-letter mutation. The new tool will allow researchers to address an additional 48% of these types of diseases.
The type of mistake that can be targeted by the new base editor is “by far, the most common kind” in people “and probably all living systems,” Liu said.
The team’s new base editor can fix these genetic errors by rearranging the atoms in a single faulty A and turning it into an I. The editor can also alter the T that was paired with the original A in the double-stranded helix of DNA and turn it into a C, Liu said.
Like the previously described base editing system, the new editor relies on the CRISPR-Cas9 complex to locate a specific sequence of bases within a genome and bond to it. Normally, CRISPR-Cas9 would then make a double cut in the DNA and either insert or delete genetic information, but Liu’s group uses a crippled form of CRISPR that can’t make a cut.
Instead, it pulls the DNA strand away from its partner, allowing an enzyme attached to the CRISPR system to change the base at the target site.
Although Liu and his lab partners had successfully engineered one base editor before publishing their most recent work, they faced fresh challenges when they set about creating an editor that could alter an adenine.
In previous work, the team took gene-editing tools found in nature and then synthesized them to create a targeted single base editor.
Unfortunately, nature doesn’t make an enzyme that can convert an A to an I in DNA. That meant they had to evolve one in the lab.
“We have an 18-year rule in our laboratory, unbroken until now, that it is unwise to embark on a project in which the first step requires the evolution of the starting material for steps 2 to 20,” Liu said. “But in this case, we thought the potential usefulness of an adenine base editor was worth the risk.”
It took more than two years, but ultimately, they were successful. After lots of trial and error, first author and post doctoral fellow Nicole Gaudelli was able to generate an enzyme that can convert AT base pairs to GC base pairs in human cells with an average efficiency of 53% and almost no errors.
It’s not perfect, but it’s a vast improvement over other methods currently in use to address point mutations.
In the paper, the authors compared their adenine base editor with the more traditional gene editing approach known as homology directed repair, or HDR. They report that their new tool was about 10 times more efficient than HDR, and resulted in at least 100 times fewer undesired products like random insertions or deletions.
The researchers also offered a glimpse at how their editor might be used in the future to combat genetic diseases.
In one experiment, they went after a point mutation that is a common cause of hereditary hemochromatosis, which causes an excessive build-up of iron in a patient’s blood that can be fatal. Using their adenine base editor, they were able to correct the mutation in cells derived from a patient with HHC.
In a second example, the team used its new base editor to install a pair of mutations that activate genes that code for the production of fetal hemoglobin. These genes are usually silenced around birth, but they can be used to protect against certain blood diseases like sickle cell anemia if they are allowed to remain active through adulthood.
These early demonstrations are promising, but Liu cautioned that base editors will not be used to address genetic diseases in living humans any time soon. (The aforementioned experiments were all done on cells grown in petri dishes).
Before that can happen, researchers will have to determine the best way to deliver the base editor machinery to the right tissues in the body and into the right cells. They will also have to figure out when in a patient’s life is the best time to deliver a certain gene therapy. To that end, his lab is collaborating with other labs that have expertise in genetic diseases.
“A tremendous amount of work is needed before this molecular machine can be used to treat diseases in humans,” he said. “But having a machine is an important starting point.”
The new base editing technique was reported on the same day that scientists from MIT and the Broad Institute announced a method for editing RNA, the molecules that translate DNA’s instructions into protein production.
By editing the letters in RNA, scientists hope to turn the protein-production machinery of certain cells on and off without making permanent changes to a person’s genetic code. The technique, which goes by the acronym REPAIR and is described in the journal Science, has the potential to treat diseases of the brain, muscles, liver and kidney as well as cancer and autoimmune disorders.